Search Results
Results for: 'covalent bonds'
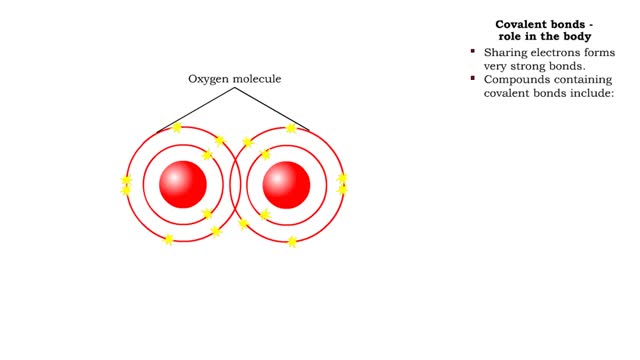
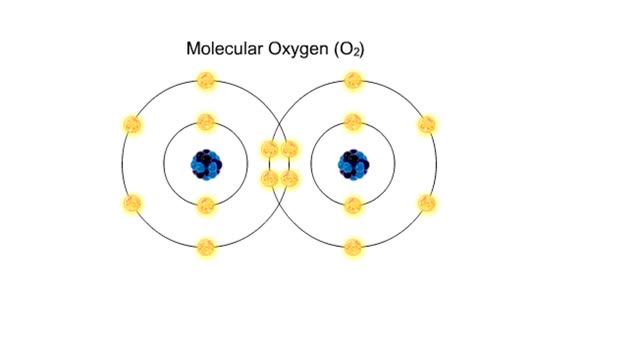
Covalent bonds - role in the body
By: HWC, Views: 6640
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
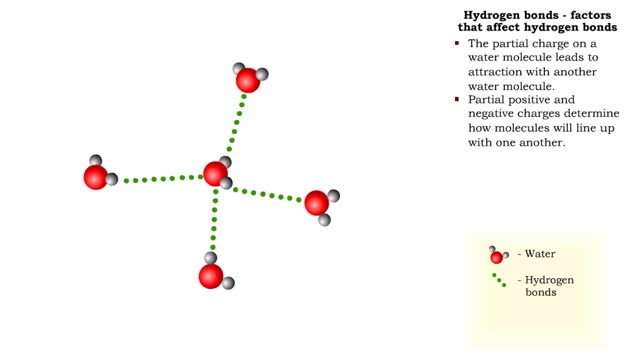
Hydrogen bonds - role in the body
By: HWC, Views: 6999
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
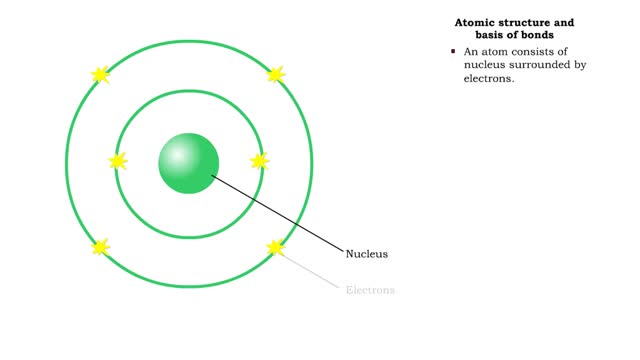
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 7025
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 3970
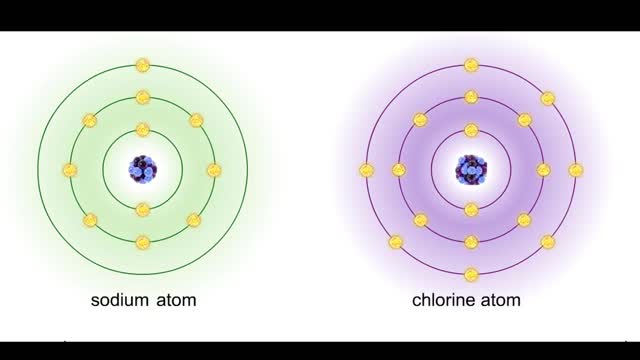
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
By: HWC, Views: 5455
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
By: HWC, Views: 6065
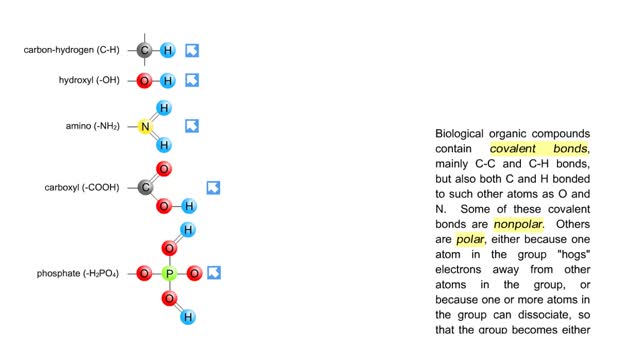
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 5903
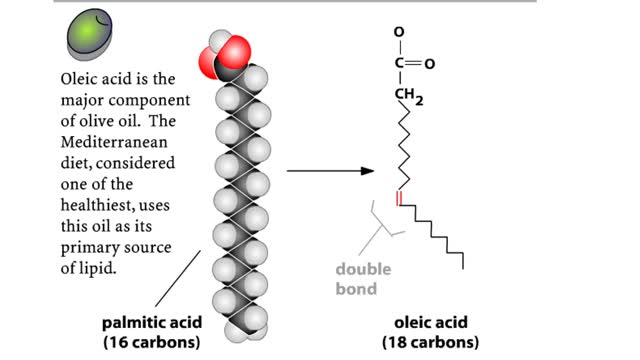
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
By: HWC, Views: 6468
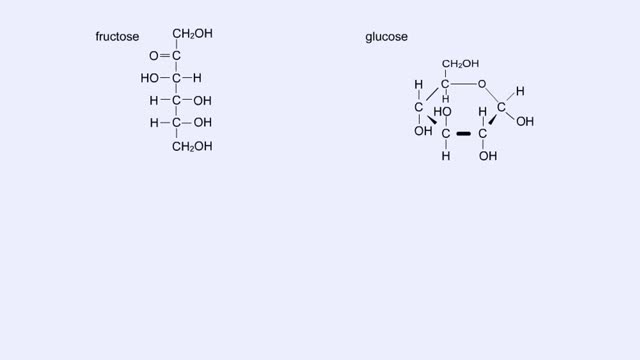
Here are the molecular structures of three simple sugars: glucose, ribose, and fructose. Look at these simple sugars and identify what characteristics they all share. As you can see, all of the carbohydrates have carbon, hydrogen, and oxygen in a ratio of 1:2:1 and there is always a double bo...
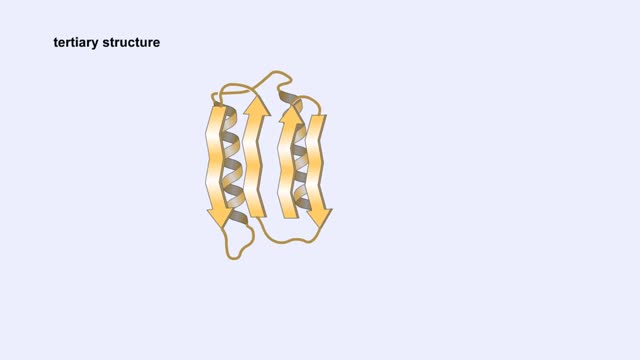
Protein Structure - Primary, Secondary, Tertiary and Quaternary
By: HWC, Views: 6632
A protein's first order structure, or primary structure, begins with the amino acid sequence of the polypeptide chain. The 20 different amino acids can be arranged in an infinite number of sequences. For example, the hormone insulin, which regulates the uptake of glucose from the blood into ce...
Advertisement